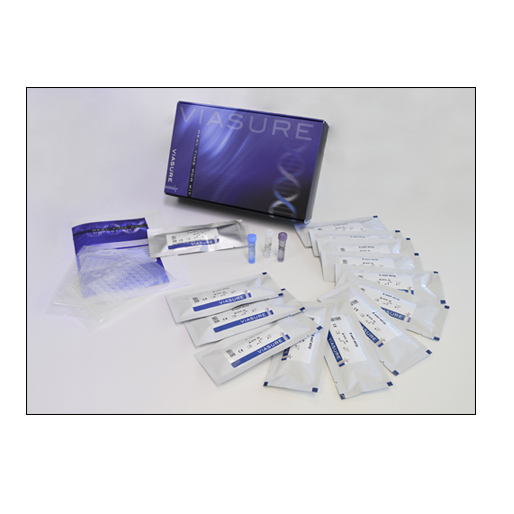
Certest™ VIASURE Respiratory Panel IV Real Time PCR Detection Kit
On demandCertest™ VIASURE Respiratory Panel IV Real Time PCR Detection Kit
VIASURE Respiratory Panel IV Real Time PCR Detection Kit is designed for the specific identification and differentiation of Influenza A and Influenza B (Flu A and/or B); Influenza A (H1N1)2009; Respiratory Syncytial Virus type A and/or B (RSV A and/or B); Parainfluenza 1 (PIV-1), Parainfluenza 2 (PIV-2), Parainfluenza 3 (PIV-3) and/or Parainfluenza 4 (PIV-4); human Adenovirus (AdV), Metapneumovirus (MPV) and/or Bocavirus (BoV); Human rhinovirus (HRV) and/or human enterovirus (HEV); and/or ); Coronavirus (CoV) 229E, NL63, OC43 and/or U1
strains in respiratory samples from patients with signs and symptoms of respiratory infection.
This test is intended for use as an aid in the diagnosis of Flu A and/or B; Influenza A H1N1)2009; RSV A and/or B; Parainfluenzas viruses; AdV, MPV and/or BoV, HRV and/or HEV; CoV 229E, NL63, OC43 and/or HKU1 strains in combination with clinical and epidemiological risk factors.
RNA/DNA are extracted from clinical specimens, multiplied using Real Time amplification and detected using specific primers and a fluorescent reporter dye probe for Flu A and B; Influenza A (H1N1)2009; RSV A and B; Parainfluenza 1, Parainfluenza 2, Parainfluenza 3 and Parainfluenza 4; AdV, MPV and BoV, HRV and HEV; CoV 229E, NL63, OC43 and HKU1 strains.
Contents
- Respiratory Panel IV 8-well strips: A mix of enzymes, primers-probes, buffer, dNTPs, stabilizers and Internal control in stabilized format, 6/12 x 8-well strip
- Rehydration Buffer: Solution to reconstitute the stabilized product, 1 vial x 1.8 mL
- Respiratory Panel IV Positive Control: Non-infectious synthetic lyophilized cDNA, 1 vial
- Negative Control: Non template control, 1 vial x 1 mL
- Water RNAse/DNAse free: Water RNAse/DNAse free, 1 vial x 1 mL
There are no specifications
There are no report
You May Also Like
Certest™VIASURE SARS-CoV-2 (ORF1ab and N genes) Real Time PCR Detection Kit 2ml Tube (4 tubes x 24rxn)
$ 431
In stock
Certest™ VIASURE Leishmania Real Time PCR Detection Kit 12 x 8-well strips, Low Profile
$ On demand
On demand
Certest™ VIASURE Respiratory Panel I Real Time PCR Detection Kit 96-well plate, High Profile
$ On demand
On demand

Certest™ VIASURE Human Metapneumovirus Real Time PCR Detection Kit 12 x 8-well strips, High Profile
$ On demand
On demand
Certest™ VIASURE Salmonella, Campylobacter & Shigella/EIEC Real Time PCR Detection Kit 6 x 8-well strips, High Profile
$ On demand
On demand
Certest™ VIASURE Flu Typing I (H1N1 & H3N2) Real Time PCR Detection Kit 96-well plate, Low Profile
$ On demand