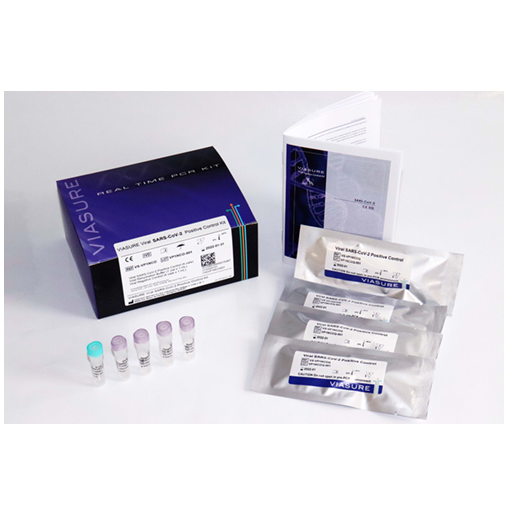
Certest™VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit
On demandCertest™ VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit is intended for monitoring the entire Real Time PCR process, from nucleic acid extraction to amplification, suitable for any RT-qPCR protocol designed for the detection of influenza A and influenza B viruses and those RT-qPCR protocols designed for the detection of the M, N, F and L genes of respiratory syncytial virus (RSV).
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit is intended for monitoring the whole reverse transcription quantitative real-time PCR (RT-qPCR) process, from nucleic acid extraction to reverse transcription and amplification steps.
VIASURE Viral Influenza A, Influenza B & RSV Positive Control Kit contains non-replicative and non-infectious Influenza A, Influenza B and Respiratory Syncytial Virus (RSV subtype A) viral particles that are expected to be processed along with respiratory samples from individuals with clinical suspicion of Influenza A, Influenza B and RSV infection.
Features
- Inactivated viral particles. Non infectious & Non replicative.
- Lyophilized presentation: transport and store at room temperature with a shelf life of 24 months.
- Monodose format for nucleic acid detection techniques.
- Final concentration range quantified by digital PCR: 11.000-110.000 copies per vial.
Content
- Viral Influenza A, Influenza B & RSV Positive Control: Non-infectious lyophilized viral particle, 4 vials
- Viral Rehydration Buffer: Solution to reconstitute the lyophilized product, 1 vial x 1mL
- Viral Negative Control: Non template control, 4 vials x 1mL
There are no specifications
There are no report
You May Also Like
Certest™VIASURE SARS-CoV-2 (ORF1ab and N genes) Real Time PCR Detection Kit 2ml Tube (4 tubes x 24rxn)
$ 431
In stock
Certest™ VIASURE Clostridium Difficile ToxB Real Time PCR Detection Kit 6 x 8-well strips, Low Profile
$ On demand
On demand
Certest™ VIASURE H. influenzae + S. pneumoniae + M. catarrhalis Real Time PCR Detection Kit 6 x 8-well strips, Low Profile
$ On demand
On demand
Certest™ VIASURE Salmonella, Campylobacter & Y. enterocolitica Real Time PCR Detection Kit 96-Well Plate, Low Profile
$ On demand
On demand
Certest™ VIASURE E. coli ETEC + EIEC Real Time PCR Detection Kit 18 x 4-well strips, Rotor Gene®
$ On demand
On demand
Applied Biosystems™ TaqMan™ Salmonella Enteritidis Detection Kit
$ On demand
On demand
Certest™ VIASURE Adenovirus, Metapneumovirus & Bocavirus Real Time PCR Detection Kit 96-well plate, Low
$ On demand