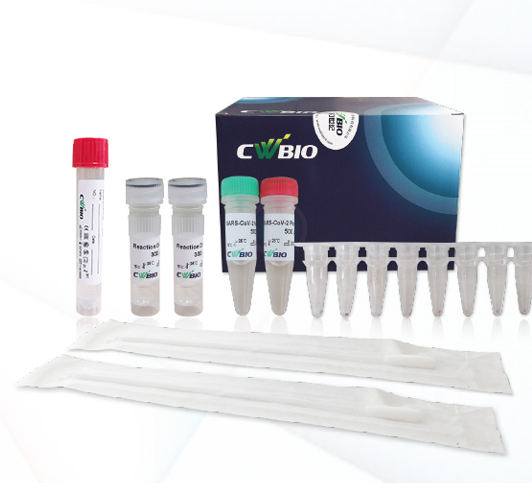
CWBio™ SARS-CoV-2 & Influenza A/B Nucleic Acid Test, 96 Tests/Box
On demand
Order number :
CW3136M
CWBio™ SARS-CoV-2 & Influenza A/B Nucleic Acid Test, 96 Tests/Box
- The SARS-Cov-2 & Influenza A/B Nucleic Acid Test is a multiplex real-time RT-PCR assay intended for simultaneous qualitative detection and differentiation of SARS-CoV-2, influenza A (IFA), and influenza B (IFB) virus RNA in upper or lower respiratory specimens (such as nasopharyngeal and oropharyngeal swabs, sputum, lower respiratory tract aspirates, bronchoalveolar lavage, and nasopharyngeal wash/aspirate or nasal aspirate) collected from individuals suspected of respiratory viral infection consistent with SARS-CoV-2, IFA or IFB by their healthcare provider. Clinical signs and symptoms of respiratory viral infection due to SARS-CoV-2, IFA or IFB can be similar
- The SARS-Cov2 & Influenza A/B Nucleic Acid Test is intended for use by trained laboratory personnel who are proficient in performing real-time RT-PCR assays. The laboratories shall have reasonable biosecurity precautions taken and protective procedures in place. The test should only be performed in laboratories that follow safety practices according to the applicable Biosafety Regulations in Microbiological and Biomedical Laboratories
- Positive results indicate active infection of SARS-CoV-2, IFA or IFB, but do not preclude co-infection with other pathogens. Clinical correlation with patient history and other diagnostic information is necessary when diagnosing patient infection status. The agent detected may not be the definite cause of disease.
Features
- After amplification, take out and put the reaction tubes/plates in a sealable plastic bag, and discard it in a designated place;
- Do not loosen the lid after amplification in case of aerosol contamination;
- Used tips shall be put directly into waste tanks containing 10% sodium hypochlorite and disposed together with other wastes;
- The experiment bench and equipment shall be decontaminated regularly by wiping with 75% alcohol and radiating with UV lamps;
- Avoid cross-contamination between reagents;
- Avoid reuse
Contents
- SF Reaction Mixture: Reverse transcriptase, hot start DNA polymerase; primer and probe sets of SARS-CoV-2, IFA, IFB and IPC; the reaction buffer, etc. (Lyophilized powder) 96 tubes
- SF Reaction Dissolvent: Tris, EDTA, etc. 1 tube (600μL/tube)
- SF Positive Control: Gene targets of SARS-CoV-2, IFA, IFB and IPC (Lyophilized powder) 1 tube
- SF Negative Control: Gene targets of IPC (Lyophilized Powder) 1 Tube
- SF Control Dissolvent: Tris, EDTA, etc. 1 tube (1mL/tube)
There are no specifications
There are no report
You May Also Like
Certest™VIASURE SARS-CoV-2 (ORF1ab and N genes) Real Time PCR Detection Kit 2ml Tube (4 tubes x 24rxn)
(Ex-work price)
$ 431
$ 431
In stock
VIASURE SARS-CoV-2 (ORF1ab and N genes) Real Time PCR Detection Kit 96-well plate, high profile
(Ex-work price)
$ 287
$ 287
In stock
VIASURE SARS-CoV-2 Triplex (ORF1ab, E & N genes) Real Time PCR Detection Kit 4 tubes x 24 Reactions
(Ex-work price)
$ On demand
$ On demand
On demand
Certest™VIASURE Flu A, Flu B & SARS-CoV-2 Real Time PCR Detection Kit 96-well plate, High profile
(Ex-work price)
$ On demand
$ On demand
On demand
Certest™ VIASURE MERS Coronavirus Real Time PCR Detection Kit 12 x 8-well strips, High Profile
(Ex-work price)
$ On demand
$ On demand
On demand
CWBio™ SARS-CoV-2 & Influenza A/B Nucleic Acid Test, 48 Tests/Box
(Ex-work price)
$ On demand
$ On demand
On demand
Certest™ VIASURE Coronavirus (229E + NL63 + OC43 + HKU1) Real Time PCR Detection Kit 96-well plate, High Profile
(Ex-work price)
$ On demand
$ On demand