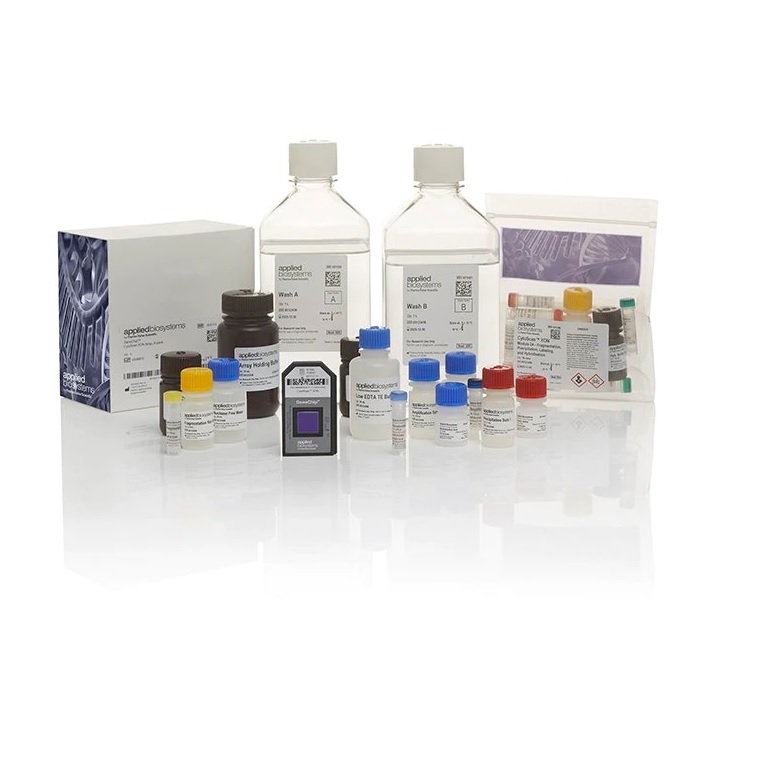
Thermo Scientific™ CytoScan™ Dx Assay Kit
On demand
Order number :
902420
Thermo Scientific™ CytoScan™ Dx Assay Kit
The American Academy of Neurology (AAN), the American College of Medical Genetics (ACMG), and the International Collaboration for Clinical Genomics (ICCG/ISCA) recommend chromosomal microarray analysis (CMA) as the first-line test to aid in the diagnostic evaluation of intellectual disability.1,2,3 The CytoScan Dx Assay is the first and only FDA-cleared chromosomal microarray test to aid in the identification of the underlying genetic cause of developmental delay, intellectual disability, congenital anomalies, or dysmorphic features in children.
Features
- First of-its-kind diagnostic test—FDA-cleared and CE marked postnatal blood test to aid in the diagnosis of developmental delay, intellectual disabilities, congenital anomalies, or dysmorphic features
- Analyze the entire genome with one test—accurately detect numerous chromosomal variations of different types, sizes, and genomic locations at higher resolution than karyotyping and more comprehensively than conventional FISH
- Designed for today and the future—the design of the CytoScan Dx Assay, which includes 2.69 million functional markers across the entire genome, ensures that most genes are represented, not only those identified as currently relevant
- Dual probe content with high-density SNPs—containing both CN and SNP probes, the CytoScan Dx Assay can elucidate allelic imbalances and identify LOH/AOH that can be associated with uniparental disomy or consanguinity, both of which increase the risk of recessive disorders. SNP patterns also provide confirmation of copy number changes.
- Exceptional performance—high specificity, sensitivity, accuracy, and resolution across the genome
- Streamlined data analysis—Chromosome Analysis Suite Dx (ChAS Dx) Software has an intuitive graphical interface for streamlined analysis workflows, ISCN 2013 array nomenclature, and links to databases* to support data analysis workflows
- Improved diagnostic yield—due to its higher resolution and whole-genome coverage, the CytoScan Dx Assay improves diagnostic yield by an incremental 12.5% enabling accurate and more effective diagnosis when compared to G-banded karyotype
There are no specifications
There are no report
You May Also Like
Applied Biosystems™ Arcturus™ Paradise™ PLUS 2 Round Kit, biotin labeling, no solvents, 48 Samples
(Ex-work price)
$ On demand
$ On demand
On demand

Applied Biosystems™ Axiom™ Wheat HD Genotyping Arrays, Array Plate B
(Ex-work price)
$ On demand
$ On demand
On demand
Applied Biosystems™ TaqMan™ Array, Human MicroRNA A Cards v2.0
(Ex-work price)
$ On demand
$ On demand
On demand

Applied Biosystems™ Axiom™ PharmacoFocus™ Assay Kit, mini 96-array
(Ex-work price)
$ On demand
$ On demand
On demand
Applied Biosystems™ TaqMan™ Array, Human Neurotransmitter, Fast 96-well
(Ex-work price)
$ On demand
$ On demand
On demand
Applied Biosystems™ TaqMan™ Array, Human Apoptosis, Fast 96-well
(Ex-work price)
$ On demand
$ On demand
On demand
Applied Biosystems™ TaqMan™ Array, Human AHR Pathway, Fast 96-well
(Ex-work price)
$ On demand
$ On demand
On demand

Applied Biosystems™ GeneChip&trade Human Gene 1.1 ST Array Plate, 24 Array Plate
(Ex-work price)
$ On demand
$ On demand