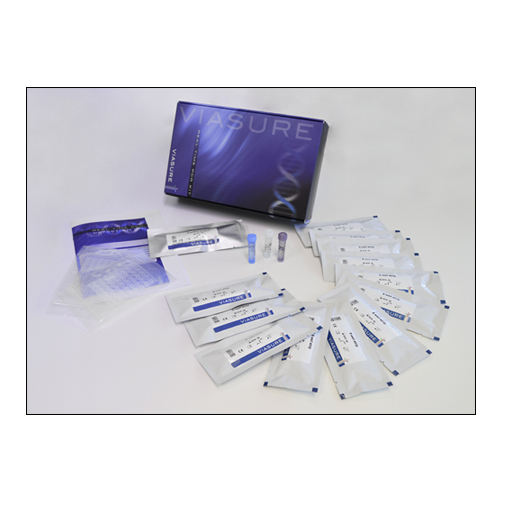
Certest™ VIASURE Respiratory Panel III Real Time PCR Detection Kit
On demandCertest™ VIASURE Respiratory Panel III Real Time PCR Detection Kit
VIASURE Respiratory Panel III Real Time PCR Detection Kit is designed for the specific identification and differentiation of Influenza A (Flu A), Influenza B (Flu B) and/or Human Respiratory Syncytial Virus (RSV); Parainfluenza 1 (PIV-1), Parainfluenza 2 (PIV-2), Parainfluenza 3 (PIV-3) and/or Parainfluenza 4 (PIV-4); human Adenovirus (AdV), Metapneumovirus (MPV) and/or Bocavirus (BoV); human rhinovirus (HRV) and/or human enterovirus (HEV); Coronavirus (CoV) 229E, NL63, OC43 and/or HKU1 strains; Chlamydophila pneumoniae, Mycoplasma pneumoniae and/or Legionella pneumophila; Haemophilus influenzae, Streptococcus pneumoniae and/or Moraxella catarrhalis in respiratory samples from patients with signs and symptoms of respiratory infection.
This test is intended to be used for research purposes, without any medical objective is not regarded as devices for performance evaluation.
RNA/DNA are extracted from clinical specimens, multiplied using Real Time amplification and detected using specific primers and a fluorescent reporter dye probe for Flu A, Flu B and RSV; Parainfluenzas viruses; AdV, MPV and BoV; HRV and HEV; CoV 229E, NL63, OC43 and HKU1 strains; C. pneumoniae, M. pneumoniae and L. pneumophila; and H. influenzae, S. pneumoniae and M. catarrahalis.
Contents
- Respiratory Panel III 8-well strips: A mix of enzymes, primers-probes, buffer, dNTPs, stabilizers and Internal control in stabilized format, 6/12 x 8-well strip
- Rehydration Buffer: Solution to reconstitute the stabilized product, 1 vial x 1.8 mL
- Respiratory Panel III Positive Control: Non-infectious synthetic lyophilized cDNA, 1 vial
- Negative Control: Non template control, 1 vial x 1 mL
- Water RNAse/DNAse free: Water RNAse/DNAse free, 1 vial x 1 mL
There are no specifications
There are no report
You May Also Like
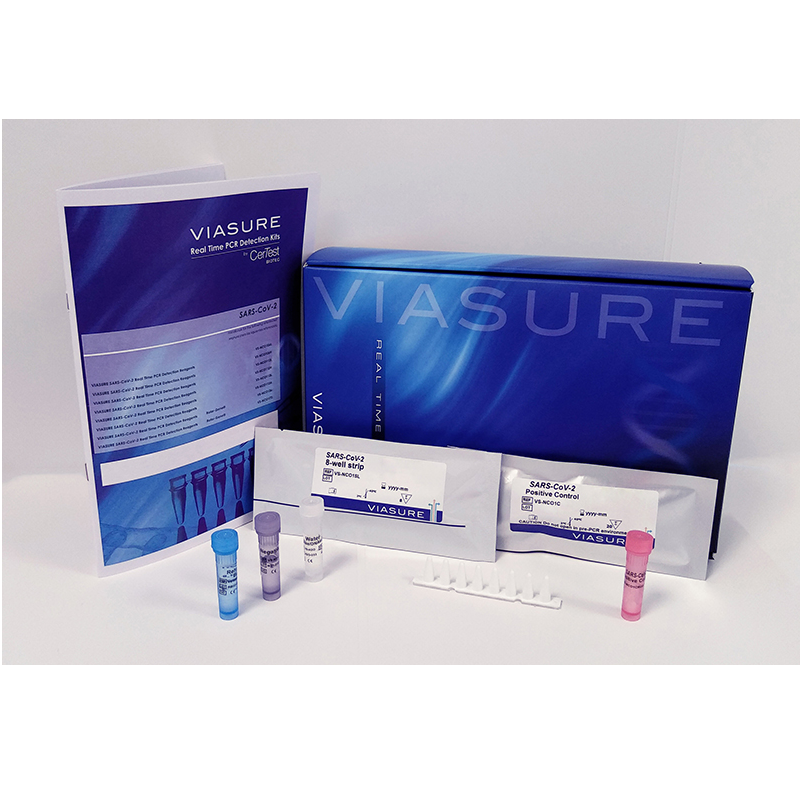
Certest™VIASURE SARS-CoV-2 (ORF1ab and N genes) Real Time PCR Detection Kit 2ml Tube (4 tubes x 24rxn)
$ 431
In stock
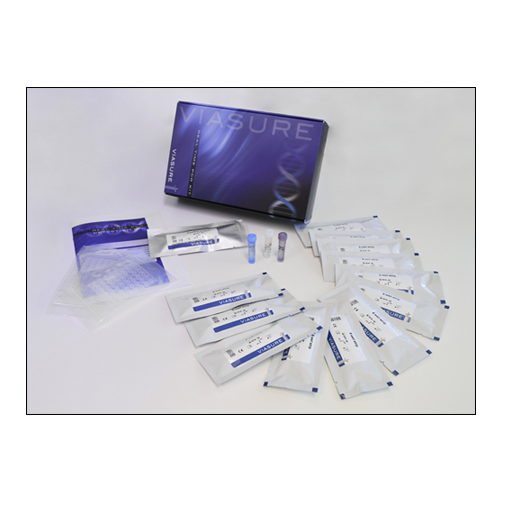
Certest™ VIASURE Salmonella, Campylobacter & Shigella/EIEC Real Time PCR Detection Kit 6 x 8-well strips, Low Profile
$ On demand
On demand
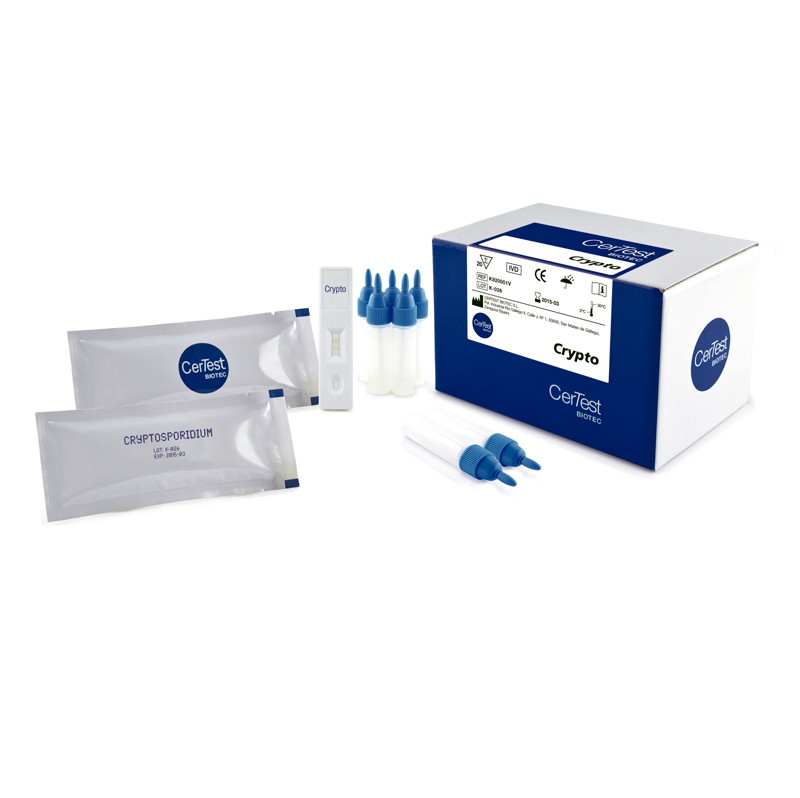
Certest™ Cryptosporidium parvum (Crypto) Fecal Antigen Rapid Test
$ On demand
On demand

Certest™ VIASURE Salmonella, Campylobacter & Shigella/EIEC Real Time PCR Detection Kit 96-well plate, High Profile
$ On demand
On demand
Certest™ VIASURE Herpes virus 1, Herpes virus 2 & Varicella Zoster Virus Real Time PCR Detection Kit 96-well plate, High Profile
$ On demand
On demand
Certest™VIASURE SARS-CoV-2, FLU & RSV Real Time PCR Detection Kit 12 x 8-well strips, High profile
$ On demand
On demand
Certest™ VIASURE M. tuberculosis complex + non-tuberculous mycobacteria Real Time PCR Detection Kit 96-well plate, Low Profile
$ On demand
On demand
Certest™ VIASURE Shigella/EIEC Real Time PCR Detection Kit 96-well plate, High Profile
$ On demand